Accuracy of Glassware
A Carolina EssentialsTM Activity

Total Time: 45-60 mins
Prep: 15 mins | Activity: 30-45 mins

Life Science, Physical Science
6-8
Middle School
- Total Time: 45-60 minutes [ Prep: 15 mins | Activity: 30-45 mins ]
- Subject: Life Science, Physical Science
- Grade: Middle School
Overview
Many science classes begin with an overview of equipment. Students memorize the names of laboratory glassware and their functions but rarely determine accuracy or relate glassware accuracy to function. In this activity, students employ density calculations to establish the accuracy of several common pieces of laboratory glassware and make a claim relating the accuracy of a piece of glassware to its designated purpose. Accuracy is defined as how close a measurement is to the true or standard value. Students compare accuracy values for different pieces of glassware using percent error calculations and use percent error as evidence for justifying a claim relating glassware accuracy to use.
Phenomenon
Students may have seen wet (or liquid) and dry measuring cups in the kitchen and wondered if a wet cup is equal in volume to a dry cup. This quick demonstration is an easy segue into the concept of laboratory glassware accuracy. Obtain dry and liquid kitchen measuring cups. The volume of the dry cup does not matter. Fill the dry cup with water, and then pour the water into the liquid measuring cup. Ask students to observe. Repeat the demonstration with a dry substance—flour, sugar, rice, and small beans are good examples. Ask students to make a claim about the accuracy of the measuring cups.
Essential Question
How does the accuracy of a piece of glassware help determine its use?
Activity Objectives
- Determine the percent error in volume for several pieces of laboratory glassware.
- Make a claim about the acceptable laboratory use of each piece of glassware based on accuracy.
Next Generation Science Standards* (NGSS)
MS-ETS1-3. Analyze data from tests to determine similarities and differences among several design solutions to identify the best characteristics of each that can be combined into a newsolution to better meet the criteria for success.
SCIENCE & ENGINEERING PRACTICES
Planning and Carrying Out Investigations
Using Mathematics and
Computational Thinking
DISCIPLINARY CORE IDEA
ETS1.B: Defining and Delimiting Engineering Problems
- There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem.
CROSSCUTTING CONCEPTS
Structure and Function
- Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem.
Materials
- Beaker
- Erlenmeyer Flask
- Graduated cylinder
- Volumetric Flask
- Electronic Balance
- Thermometer
- Container of room temperature tap water
- *Glassware may be of any size, providing the mass of the glassware plus water does not exceed the limits of the balance. The volume of the volumetric flask will determine the volume of water used for all pieces of glassware, so group the glassware appropriately.
Safety Procedures and Precautions
Remind students to be mindful of where they place glassware. Have a broom, dust pan, and a container for broken glassware available. Remind students not to pick up broken glass with their hands.
Teacher Preparation and Disposal
Group glassware prior to the activity. Make certain the volume of water the volumetric flask holds will fall between the graduations on the other pieces of glassware. No chemical or waste disposal is necessary. Dispose of any broken glass in the appropriate container.
STUDENT PROCEDURES
A. Visual Inspection
- Weigh each piece of glassware and record the mass in table B.
- As a group, begin with the volumetric flask. Record the volume printed on the flask then fill the flask with tap water so the meniscus sits on the etched line.
- Pour the water into another piece of glassware and record the volume on that piece of glassware. Estimate the volume if needed.
- Repeat step 2 for the remaining pieces of glassware.
B. Glassware Accuracy Using Density Calculations
- Each group member must collect data on every piece of glassware.
- Record the value on the volumetric flask. You will add water to that mark on each piece of glassware. _____________mL
- Add water until the volume recorded in step 2 is present in the piece of glassware being tested. Use the graduations on the glassware. Do not pour water from 1 piece of glassware to another.
- Record the temperature of the water.
- Weigh the glassware with the water and record the value.
- Repeat steps 3 to 6 for the other 3 pieces of glassware.
- Calculate the mass of water in each piece of glassware.
- Calculate the group average for the mass of water in each piece of glassware and temperature.
TEACHER PREPARATION AND TIPS
- Remind students how to read volume-level surface, eye-level, from the bottom of the meniscus.
- Water from the volumetric flask should be poured into each piece of glassware.
- Every individual student should take measurements from all pieces of glassware.
- The volume of the volumetric flask determines the volume being used in all pieces of glassware.
- Review with students that temperature is critical when working with density. Higher temperature generally means a greater volume, which decreases density.
- Mass of water = (mass of water + flask) – mass of flask or mass2 – mass1
Data and Observations
Student answers will vary.
A. Visual Inspection

B. Glassware Accuracy Using Density Calculations
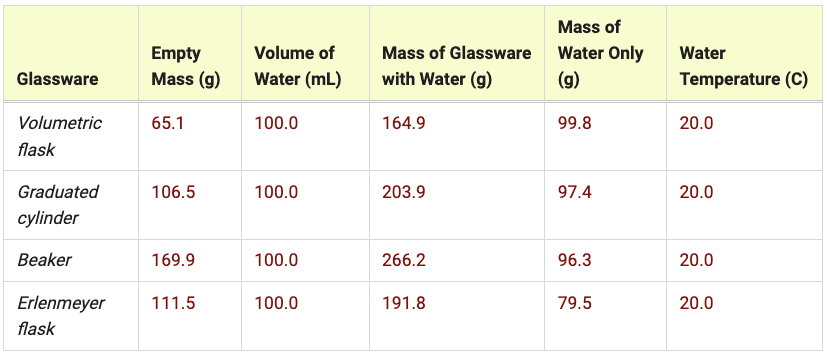
Group Averages

Analysis & Discussion
Based on the data collected, rank the 4 pieces of glassware from most accurate to least accurate. Explain your reasoning.
Student answers will vary, but they should notice higher accuracy for the volumetric flask and graduated cylinder.
Using individual data and the density of water table, calculate the actual volume of water in each piece of glassware.
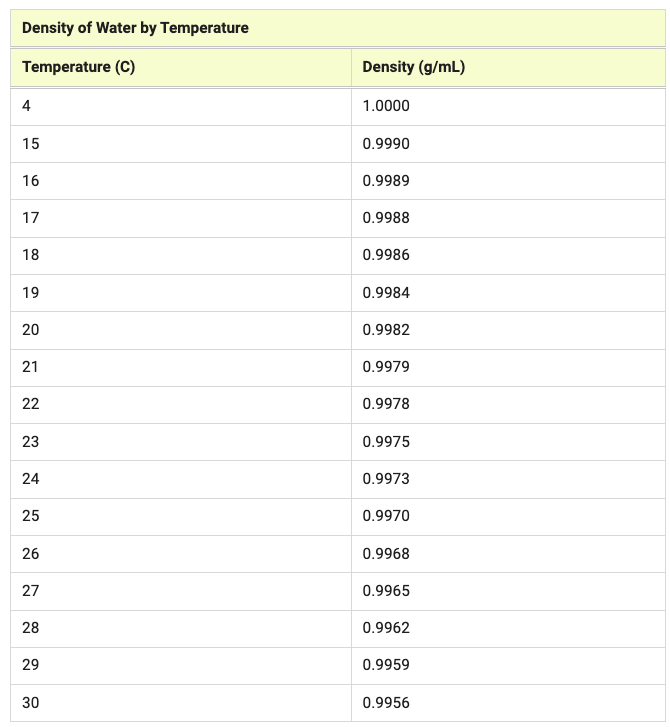
Density = mass/volume
-
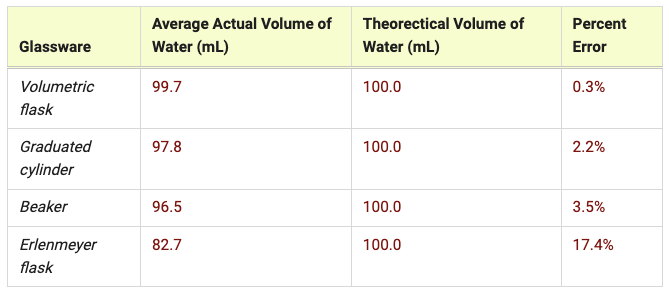
Average the individual actual volumes for each piece of glassware and place the values in the table.
-
Calculate the percent error for each piece of glassware and record the values.

-
Based on percent error only, rank the glassware from most accurate to least accurate. Use your data to explain your ranking.
Volumetric flask, graduated cylinder, beaker, flask
The volumetric flask had the lowest percent error (0.3%), so it is the most accurate. The graduated cylinder and beaker were similar in percent error (2.2% and 3.5% respectively). Both were small, so they are fairly accurate. The flask had the highest percent error (17.4%), so it is the least accurate.
-
How do the 2 methods of determining accuracy compare? Were the results the same or different?
The rankings were the same. The first method provided a less precise ranking of glassware since the volume of water had to be estimated for every piece of glassware but the volumetric flask. The second method was more precise because volume was calculated using density, the temperature of water, and a digital balance.
-
Based on accuracy, assign each piece of glassware appropriate uses and state evidence for your choice. For example, which pieces should be used for measurement, and which should not be used for measurement?
The volumetric flask is the most accurate, but it only measures 1 volume—in this case, 100mL. If you need to measure other volumes besides those on volumetric flasks, then the graduated cylinder is the best tool. The beaker is pretty accurate, and you can easily stir and heat chemicals in it. I would not use a flask to measure volume given the large percent error. If volume only had to be estimated, a flask would be OK. It is also good for mixing and heating.
SHOP THE KIT
SAFETY REQUIREMENTS
- Safety Goggles Required
REFERENCE KITS
HELPFUL LINKS
VIEW MORE ESSENTIALS
*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of, and do not endorse, these products.







