Electron Configuration and Periodic Trends
A Carolina EssentialsTM Activity

Total Time: 60 mins
Prep: 15 mins | Activity: 60 mins

Physical Science, Chemistry
9-12
High School
- Total Time: 60 minutes [ Prep: 15 mins | Activity: 60 mins ]
- Subject: Physical Science, Chemistry
- Grade: High School
Overview
Students are often asked to memorize the periodic trends found on the periodic table. With a data-driven approach, students analyze data patterns to identify the trends themselves. Relating periodic trends to atomic structure and electron arrangement provides an explanation for the trends, allowing students to construct their own data-based explanation. This activity may be assigned as homework or used as a group activity in class. Revisit the data table during units on bonding and chemical reactivity as evidence for additional explanations.
Phenomena
How did we get from Mendeleev’s version of the periodic table to Seaborg’s, and then to the current version of the periodic table?

Essential Question
How can the periodic table be used to predict relative properties of elements?
Activity Objectives
- Relate electron configurations to periodic properties.
- Identify the trend for several periodic properties.
Next Generation Science Standards* (NGSS)
PE HS-PS1-1. Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy levels of atoms.
SCIENCE & ENGINEERING PRACTICES
Developing and Using Models
- Use a model to predict the relationships between systems or between components of a system.
DISCIPLINARY CORE IDEA
PS1.A: Structure and Properties of Matter
- Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons.
- The periodic table orders elements horizontally by the number of protons in the atom’s nucleus and places those with similar chemical properties in columns. The repeating patterns of this table reflect patterns of outer electron states.
CROSSCUTTING CONCEPTS
Patterns
- Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for the causality in explanations of phenomena.
Materials
- Student activity pages
Safety Procedures and Precautions
No PPE is required for the activity.
Teacher Preparation and Disposal
Copy or upload the student page to a class web site for student access.
SCIENCE & ENGINEERING PRACTICES
- Complete the electron configuration for the first 18 elements on the periodic table.
- Convert the electron configuration to a noble gas configuration.
- Use the noble gas configuration to determine the number of valence level electrons for each element.
- Use the number of valence level electrons to determine the ionic charge of each element.
- Graph the first ionization energy (y-axis) against atomic number (x-axis).
- Graph electronegativity (y-axis) against atomic number (x-axis).
- Graph atomic radius (y-axis) against atomic number (x-axis).
TEACHER PREPARATION AND TIPS
- Review procedures for writing electron configurations with students. If this has not been taught yet, use the teacher guide and give students the configurations. They can still identify and explain patterns.
- You may wish to have students complete this activity in groups of 3 and assign a graphing procedure to each of the students.
- The x-axis is atomic number. You may wish to have students label the x-axis with atomic symbol.
- It is beneficial to have a class discussion about each graph before the discussion questions are answered.
Data and Observations

1. Graph of first ionization energy

2. Graph of electronegativity

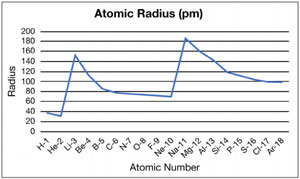
3. Graph of atomic radius
Analysis & Discussion
1. Describe and explain the pattern in electron configurations for the first 18 elements.
The first 2 elements fill the 1s level. Elements 3 to 10 fill 1s, 2s, and 2p, totaling 8 electrons for the level. Elements 11 to 18 follow the same pattern as elements 3 to 10, except electrons are placed in the third level instead of the second level. Eight electrons need to be added to the third level to complete it.
2. Describe and explain the pattern in the number of valence level electrons for the first 18 elements.
The first row, H and He, has 1 and 2 valence level electrons. Level 2, elements 3 to 10, have 1 to 8 valence level electrons respectively. Level 3, elements 11 to 18, follow the same pattern as level 2—1 to 8 valence level electrons.
3. Describe and explain the pattern in ionic charge for the first 18 elements.
Elements with 1 valence level electron (H, Li, Na) have an ionic charge of 1+ and lose 1 electron. They are in column 1 of the periodic table. Excepting helium, elements with 2 valence level electrons (Be and Mg) have an ionic charge of 2+ and lose 2 electrons. They are in column 2 of the periodic table.
B and Al have 3 valence level electrons, an ionic charge of 3+, and lose 3 valence level electrons. They are in column 13. C and Si have 4 valence level electrons, which means they can gain 4 electrons or lose 4 electrons. They have an ionic charge of 4- or 4+ respectively. They are in column 14 of the periodic table.
N and P have 5 valence level electrons, so they will likely gain 3 electrons, resulting in a 3- charge. There are exceptions to this. Nitrogen can lose all 5 electrons, resulting in a 5+ charge. They are in column 15 on the periodic table. O and S gain 2 electrons, resulting in a 2- charge, and are found in column 16. F and Cl follow the same pattern. They lose a single valence electron, giving a 1- charge, and are in column 17.
He, Ne, and Ar are all found in column 18. Ne and Ar have a full valence level with 8 electrons, so they will neither gain nor lose electrons. He has a full valence level with just 2 electrons, since all of its electrons are in level 1. It will neither gain nor lose electrons because its valence level is also full. Since He, Ne, and Ar all have full valence levels and do not gain nor lose electrons, they have an ionic charge of 0.
The general pattern for ionic charge going horizontally across a period is 1+, 2+, 3+, 4+, 4-, 3-, 2-, 1-, 0. If electrons are lost, there is a positive ionic charge. If electrons are gained, there is a negative ionic charge.
4. Explain how ionic charge is related to the number of valence level electrons.
The number of electrons lost or gained determines the magnitude of the charge. If electrons are lost, the charge is positive, and if electrons are gained, the charge is negative.
5. Describe and explain the pattern in first ionization energy for the first 18 elements.
First ionization energy increases across a period (row) and decreases down a group (family or column).
6. Explain how ionization energy is related to electron configuration.
The larger the positive charge of the nucleus is, (more protons), the greater the nuclear charge and attraction for electrons. Removing an electron requires more energy. As an electron gets further from the nucleus, the weaker the nuclear attraction becomes, requiring less energy to remove an electron. This is called nuclear shielding.
7. Describe and explain the pattern in electronegativity for the first 18 elements.
Electronegativity increases across a period and decreases down a group.
8. Explain how electronegativity is related to electron configuration.
As the valence level fills with electrons, it requires more energy to attract bonding pairs of electrons because there must be enough energy to overcome the repulsion of electrons already in the valence level. The noble gases do not have electronegativity values because they have a complete valence level.
9. Describe and explain the pattern in atomic radius for the first 18 elements.
Atomic radius decreases across a period and increases down a group.
10. Explain this trend.
As you go across a period from left to right, the nuclear charge increases so the valence level electrons are attracted more strongly to the nucleus, decreasing the distance from the nucleus, which in turn decreases the atomic radius.
11. Explain to Mendeleev, in 3 to 4 sentences, how and why the periodic table has changed.
The periodic table has changed in 2 major ways since Mendeleev constructed the first periodic table.
First, there are many more elements now. Even though Mendeleev predicted the existence of yet undiscovered elements, much of what has been added to the periodic table are synthetic or man-made elements. The technology to produce elements was not available in Mendeleev’s time.
Second, the major organizing principle has changed. Mendeleev organized the periodic table by atomic mass. We now have a better understanding of atomic structure, so the modern organizing principle is atomic number. With the addition of quantum mechanics and a deeper understanding of electron behavior, elements are also located on the periodic table based on electron configuration. Seaborg made the last major change to the periodic table when he proposed the f-block elements of the lanthanide and actinide series.
SHOP THE KIT
REFERENCE KITS
HELPFUL LINKS
VIEW MORE ESSENTIALS
*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of, and do not endorse, these products.










