Crystallization Investigation

Crystallization through precipitation lab Crystals are solids that form when molecules join in a regular repeating pattern. Crystals mesmerized humans early in our history and served as currency as well as decoration. Today, the jewelry industry relies on precious and semi-precious crystals for rings, pendants, and more; the electronics industry relies on quartz crystals for […]
Color Change Chemical Reaction

Also known as the traffic light reaction, indigo carmine produces a colorful demonstration In this activity, a redox indicator (indigo carmine) changes color as a result of electron transfer. The introduction of oxygen through swirling causes the indigo carmine to turn green as it is oxidized. Upon standing, the indigo carmine is reduced by the […]
Cooking Eggs with Chemicals
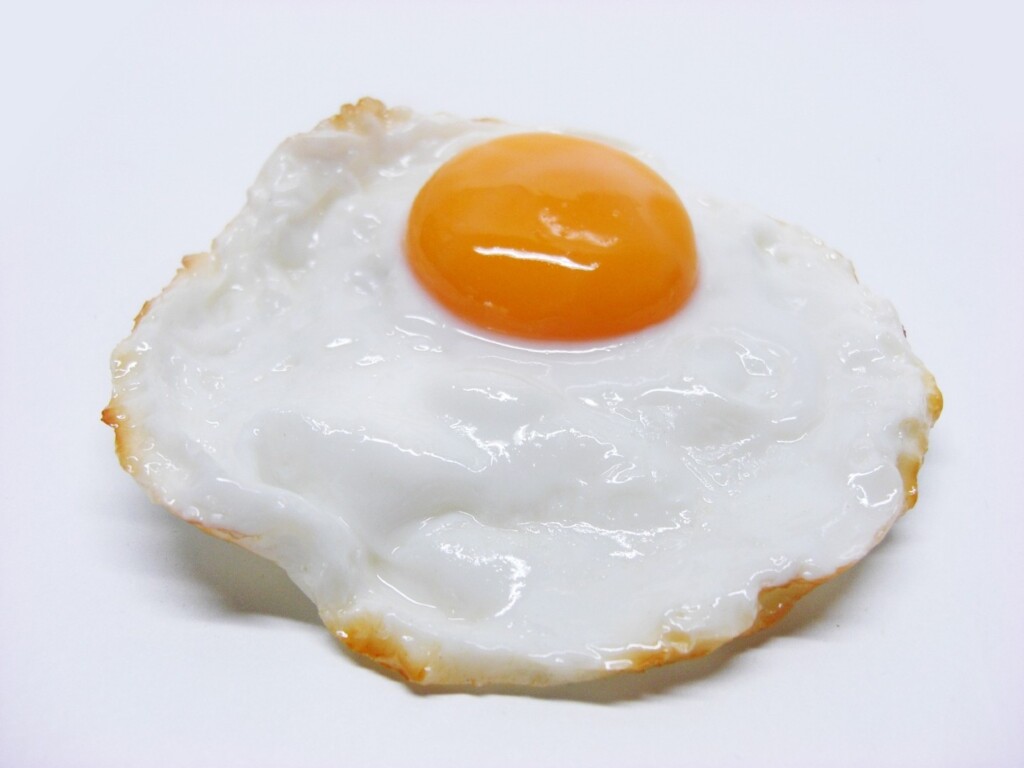
A demonstration of denaturing protein Introduce high school biology and chemistry students to the concept of denatured proteins with this inexpensive, fun demonstration. Your students will be amazed when you “cook” an egg in hydrochloric acid, and they will learn that a protein (egg white) can be denatured by heat, or, as shown in the […]
Single-replacement Reaction

Create silver decorations with chemistry In this activity students perform a redox reaction in which silver take the place of copper in copper wire. The copper changes from its elemental form to its aqueous ionic form. During the course of the reaction, the silver ions are reduced and are removed from the solution. The silver […]
Make Your Own Indicators

Testing the pH of a solution is a fundamental skill in chemistry. Students typically test pH using a pH meter, litmus or pH paper strips, or one of a variety of commercial acid-base indicator solutions. Acid-base indicators are weak organic acids that change color depending on the pH of a solution. A wide variety of […]
Balancing Chemical Equations

Using Models to Simplify Balancing Equation To balance chemical equations, students must know how to count atoms, understand the difference between coefficients and subscripts, and identify reactants and products. Balancing equations reinforces understanding of the law of conservation of mass as students keep in mind that the same number and types of atoms must be […]
Mole Day (October 23) Celebration

Mole Day Celebration Happy Mole Day. It’s party time! Break out the . . . the . . . the what? How do you celebrate the value of the Avogadro constant and why would you want to anyway? Keep reading and we’ll give you all kinds of ways to commemorate the big day. As for […]
Rock Candy: An Edible Study of Crystallization

An Investigation of Supersaturated Solutions Making rock candy is a safe way to introduce students to solutions and crystal growth–and you can make it a tasty treat at the same time! This activity helps students visualize how a supersaturated solution grows the extra-large crystals of sucrose needed to make rock candy. Table sugar (sucrose) and […]
Classifying Chemical Reactions
Writing and balancing chemical equations is an essential skill for chemistry students, who must learn to predict the products of a reaction when given only the reactants. This becomes much easier for students to do when they learn the pattern of 5 basic categories of chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion. Types of […]
Silvering Glass

Create Silver-Mirrored Christmas Ornaments with a Redox Reaction Create silver ornaments with your students using a chemical process similar to antique mirroring techniques. In this festive activity, silver is plated onto the interior surface of a glass vial via a redox reaction. In the reaction, dextrose is oxidized to gluconic acid, and silver ions are […]
