Thermochemistry: An Endothermic Reaction
A Carolina EssentialsTM Demonstration

Total Time: 10 mins
Prep: 15 mins | Activity: 10 mins

Physical Science
6-12
Middle/High School
Overview
In this thermochemistry demonstration, students observe an extreme, spontaneous endothermic reaction between 2 solid compounds, measure changes in temperature, and make observations. The demonstration may be used with physical science or chemistry students as an introduction to thermochemistry or as an introductory example of enthalpy calculations for chemistry students. Prior to the demonstration, students can review reaction writing and product prediction.
Phenomenon
Relate heat to motion in a very simple demonstration. Ask students to quickly rub their hands together for 20 to 30 seconds. On the macroscopic scale, ask them to discuss what happened and why. Then ask if they think the same phenomenon may apply on the microscopic scale.
Essential Question
How does energy flow during a chemical reaction?
Demonstration Objectives
- Observe an endothermic reaction.
- Describe the flow of heat energy during the reaction.
- Construct an energy diagram for the reaction.
Next Generation Science Standards* (NGSS)
PE HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
SCIENCE & ENGINEERING PRACTICES
Constructing Explanations
- Students will observe an endothermic reaction and explain the energy differences between reactants to products.
DISCIPLINARY CORE IDEA
DCI: Physical Sciences-Energy
- Students will explain the flow of energy between the reaction process and the surroundings for an endothermic reaction.
CROSSCUTTING CONCEPTS
Concept: Energy and Matter: Flows, Cycles, and Conservation.
- Students will explain the difference in energy between reactants and products and the flow of energy into and out of the surroundings.
Materials
Safety and Disposal
This reaction produces ammonia gas and should be performed in a fume hood. If no fume hood is available, perform in a large, well-ventilated area and stir the contents of the beaker at a safe distance from your nose. An alternative venting method is to use a faucet aspirator connected to an inverted funnel over the reaction beaker.
Follow all federal, state, and local regulations as well as school district guidelines for the disposal of laboratory wastes. The activity involves barium, which requires special handling.
Procedures
STUDENT PROCEDURES
- Weigh 32.0 g of Ba(OH)2 and place it in the beaker.
- Weigh 11.0 g of ammonium chloride. Leave it in the weigh boat.
- Pour enough tap water on the wood block, (about 2 mL), to create a small puddle.
- Place the wooden block in a fume hood or on a lab bench in a well ventilated room.
- Turn on the fume hood.
- Take the temperature of the beaker contents.
- Put on temperature-resistant gloves
- Add 11.0 g of ammonium chloride to the barium hydroxide octahydrate.
- Set the beaker at the center of the water puddle on the block.
- Hold the beaker with one hand while you stir the two solids with a stirring rod.
- Take the temperature of the beaker contents 3-4 times during the reaction process, which takes 3-5 minutes.
- Grasp the top of the beaker and lift it. The block will be lifted as well.
TEACHER PREPARATION AND TIPS
- Allow students to examine both reactants (barium and ammonium chloride) before beginning.
- Review or introduce the terms exothermic and endothermic.
- Introduce the term enthalpy.
- Use a beaker with a flat bottom.The bottom must make complete contact with the puddle.
- For the block, a piece of 1″ × 4″ unfinished pine, cut 2 inches wider than the beaker, works well.
- Record the temperature on the board.
- Winter gloves work well.
- Ammonia gas will be generated. Ask if any student can identify the smell. If not, inform them that ammonia is a product.
- Make sure the puddle covers the entire area of the beaker bottom.
- Record the temperatures on the board.
- Look for the formation of ice on the board and a temperature in the beaker below -5.0 °C.
- Have students record observations. You may want to walk around the classroom for students to observe the ice formation.
Data and Observations

Analysis & Discussion
Physical Science
Fill in the blank in the title.
Endothermic
Was the reaction exothermic or endothermic? What data support your answer?
Endothermic because the temperature decreased
Did energy flow from the system (beaker with chemicals) to the environment or from the environment to the beaker? Explain your thinking.
Energy flowed from the environment to the system. I know this because the temperature decreased.
Did the products or the reactants have more energy? Explain.
The products have more energy than the reactants. To obtain the additional energy, energy is transferred from the surroundings, including the liquid water on the board, to the products, resulting in the environment cooling.
Balance the reaction.
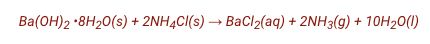
Chemistry
Draw an energy diagram for the reaction progress.

Calculate ΔH for the reaction. Show your work.

ΔH = ∑Hproducts – ∑Hreactants
ΔH = +164kJ/mole
SHOP THE KIT
SAFETY REQUIREMENTS
- Safety Gloves Required
- Safety Goggles Required
HELPFUL LINKS
VIEW MORE ESSENTIALS
*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of, and do not endorse, these products.




