Modeling Alpha and Beta Decay
A Carolina EssentialsTM Activity

Total Time: 60 mins
Prep: 15 mins | Activity: 45 mins

Physical Science
9-12
High School
- Total Time: 60 minutes [ Prep: 15 mins | Activity: 45 mins ]
- Subject: Physical Science
- Grade: High School
Overview
Students often use balanced nuclear reactions to model changes that occur in an unstable nucleus, but that doesn’t help students assess why the nucleus is unstable initially. In this activity, students use the band of stability and neutron-proton ratios to construct a simple predictive model to determine if an isotope will decay or not decay, and if the isotope does decay, whether the process is more likely to be alpha or beta decay. The activity does not address positron emission and gamma radiation.
Students calculate neutron-proton ratios for selected isotopes, determine if the isotope is stable or unstable, find the isotope’s location on the band of stability to determine decay type, and then write balanced nuclear equations for the isotopes that decay.
Phenomenon

Nuclear medicine is a branch of medicine that relies on the proper selection of radioactive isotopes to produce scans like those above and to treat many types of cancer. Why do you think nuclear decay can be a powerful and useful medical tool? Discuss responses with your students. Some students may have personal experience with diagnostic tools or treatment.
Essential Question
How can changes in the composition of the nucleus of an atom be predicted and modeled?
Activity Objectives
- Determine if an isotope is stable or unstable.
- Construct a model that predicts the type of nuclear decay expected for unstable nuclei.
Next Generation Science Standards* (NGSS)
HS-PS1-8. Develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the process of fission, fusion, and radioactive decay.
SCIENCE & ENGINEERING PRACTICES
Developing and Using Models
- Develop a model based on evidence to illustrate the relationships between systems or between components of a system.
DISCIPLINARY CORE IDEA
PS1.C: Nuclear Processes
- Nuclear processes, including fusion, fission, and radioactive decays of unstable nuclei, involve release or absorption of energy. The total number of neutrons plus protons does not change in any nuclear process.
CROSSCUTTING CONCEPTS
Energy and Matter
- In nuclear processes, atoms are not conserved, but the total number of protons plus neutrons is conserved.
Materials
- Copies of student pages or online access
- 3 Carolina® Student Infographic Periodic Table
- 3 Calculator (optional)
Safety Procedures and Precautions
No PPE is required for the activity.
Teacher Preparation and Disposal
Copy the student pages or upload them to a class web page.
STUDENT PROCEDURES
- Calculate and record the number of neutrons and protons for all isotopes in the data table.
Protons = atomic number (Z)
Neutrons = isotope mass – atomic number - Calculate the neutron-proton ratio.
Number of neutrons = x.xx or x.xx:1
Number of protons 1
Record the ratios in the data table. - Based on the background information about neutron-proton ratios and nuclear size, highlight the stable isotopes in the data table.
- Locate each isotope on the band of stability chart to confirm your decision about nuclear stability. For isotopes that decay, identify whether the nucleus decays by alpha or beta decay. Record the decay type in the chart.
- For the isotopes that decay, write a balanced nuclear reaction.
TEACHER PREPARATION AND TIPS
- Review isotope notation with students so they recall that the top number is mass and the bottom number is charge (protons) for a block representation, and mass is the number following the element name in the long-hand notation.
- For this activity, round all calculations to 2 decimal places.
- Review the band of stability chart with students. Each small block represents a different isotope. Point out that isotopes of the same element may decay through different processes.
- Remind students that a balanced nuclear equation shows conservation of charge and mass. The top numbers (mass) on both sides of the arrow must be equal, and the bottom numbers (charge) on both sides of the arrow must be equal. Use the charge to identify the daughter nuclide.
- An interesting extension project is to assign students one of the imaging isotopes or treatment isotopes for additional research. Include how the isotope is made, how it is used or administered, and what specific diseases it is used to treat.
Data and Observations
Data Table

Band of Stability

Source: Wikimedia Commons. Table isotopes en.svg.
Balanced Nuclear Equations

Analysis & Discussion
Construct a process diagram or flow chart that can be used to identify an isotope’s stability, and if unstable, how it will decay.
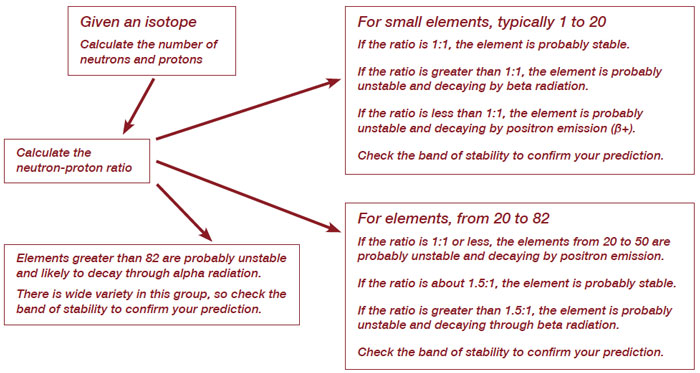
Your teacher will assign you a medical isotope. Use your model to determine if the isotope is stable or unstable, and if unstable, how it will decay. Write the balanced nuclear equation for the decay of the isotope.
Student answers will vary. Use the chart from the National Isotope Development Center (NIDC) to assign elements, and check to see that students follow their flow charts.
SHOP THE KIT
HELPFUL LINK
VIEW MORE ESSENTIALS
*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of, and do not endorse, these products.