Prep Time: 10 min
Class Time: 45 min
Students: Groups of 2
6–12 Middle / High School
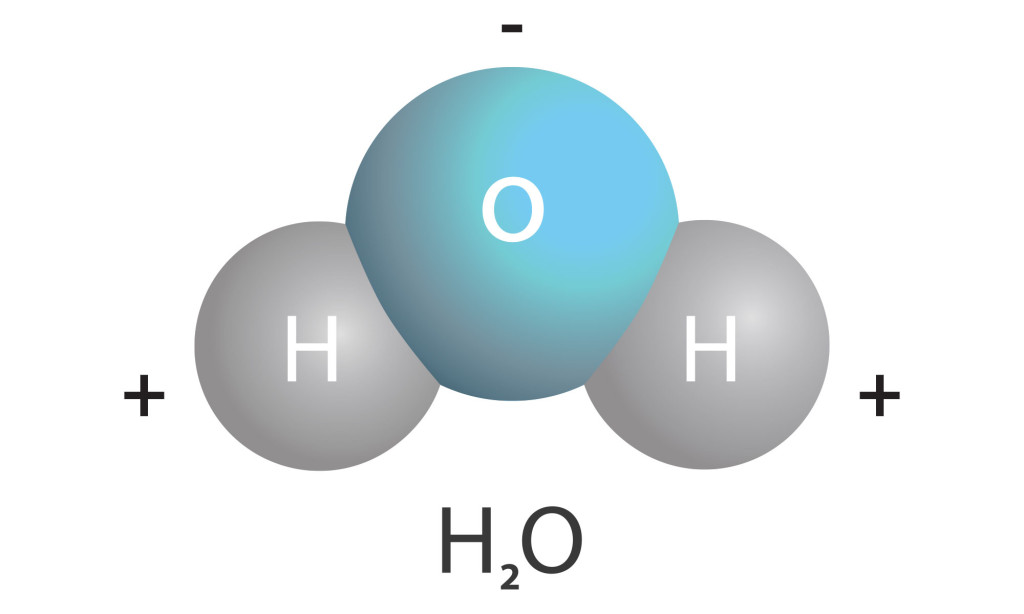
Water is a polar molecule, which means it has a positive end and a negative end. The oxygen end (blue) is like the negative pole and the hydrogen end (gray) is like the positive pole. Just like magnets, the positive end of one water molecule will attract the negative end of another water molecule resulting in the properties of adhesion, cohesion, and surface tension.
Because of water’s unique molecular properties, its observable physical properties impact our daily lives in ways we don’t often notice. This activity provides you with visual and quantitative evidence of the phenomena of adhesion, cohesion, and surface tension in water. You will compare these properties of water for deionized water and deionized water with Dawn dish detergent added to make sense of how water’s polar nature determines its physical properties.
This activity addresses the following AP® Biology concepts:
SYI-1 Living systems are organized in a hierarchy of structural levels that interact.
SYI-1.A Explain how the properties of water that result from its polarity and hydrogen bonding affect its biological function.
SYI-1.A.3 The hydrogen bonds between water molecules result in cohesion, adhesion, and surface tension.
Preparation
Students should see that they are able to transfer the distilled water but are able to transfer little to no Dawn solution. In the Dawn solution, the soap disrupts the bonds that allow the water to adhere to the side of the straw. Because of this, the solution is unable to be transferred. Note: Some cohesion plays a role in the water remaining in the straw.
Students should see that more drops are able to remain on the penny with distilled water than the penny with Dawn solution. In testing, there were about half as many drops able to remain on the Dawn solution penny. This is because, in the Dawn solution, the soap disrupts the bonds that allow the water molecules to stick to each other through cohesion. Note: Some adhesion plays a role in the water sticking to the penny but does not account for the total amount of water.
Students should see that the pencil lead is able to float on the surface of the distilled water but is not able to float on the surface of the Dawn solution. The soap in the Dawn solution disrupts the bonds between the water molecules that create surface tension.
AP® is a trademark registered by the College Board, which is not affiliated with, and does not endorse, this product.
Carolina is teamed with teachers and continually provides valuable resources–articles, activities, and how-to videos–to help teachers in their classroom.
Get the latest news, free activities, teacher tips, product info, and more delivered to your inbox.