9-12 High School
This activity serves as a student-based or teacher-led phenomenon that will lead to making sense of how reaction rates can be changed. Three factorsconcentration, temperature, and particle sizeare observed for their effect on reaction rates. In student groups or through a teacher demonstration, the 3 factors are manipulated (9 total reactions) and simple time measurements recorded.
The activity is introductory and not meant to answer the how-or-why questions of variable reaction rates but simply to let students observe and gather evidence that reaction rates can be manipulated. The materials used, Alka-Seltzer® and water, are probably familiar to many students, so they can concentrate on how the reaction rate changes and not on the reaction itself. Time measurements are made using the disappearance of the antacid and the appearance of carbon dioxide bubbles. In a quantitative lab, change in concentration of either a reactant (disappearance) or product (appearance) would be measured over time.
Ask students these questions and allow for brief discussion in groups before beginning the demonstration.
Can the rate at which a reaction occurs be changed?
HS-PS1-5. Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
Constructing Explanations and Designing Solutions
PS1.B: Chemical Reactions
Patterns
Students should wear safety goggles to prevent splash hazards.
Pre-cut and grind the Alka-Seltzer® tablets prior to the demonstration to save time. All reaction products may be disposed of down the sink.
| Temperature and Time(s) | Concentration and Time(s) | Particle Size and Time(s) |
|---|---|---|
| Room | Whole | Whole |
| Warm | Half | Chunks |
| Cold | Quarter | Powder |
| Temperature and Time(s) | Concentration and Time(s) | Particle Size and Time(s) |
|---|---|---|
| Room | Whole | Whole |
| Warm | Half | Chunks |
| Cold | Quarter | Powder |
State the relationship between each variable and reaction time.
As the temperature went up, the time decreased so the rate increased. As the amount of Alka-Seltzer® decreased, the time increased so rate decreased. As the particle size got smaller, the time decreased so the rate increased.
Use a series of particle diagrams to illustrate the variable you were assigned and the relationship between the variable and reaction rate you discovered. Use a single symbol for Alka-Seltzer® and a single symbol for carbon dioxide. You do not have to show every atom. Color or symbol code the particles.
There is a 1:3 ratio of Alka-Seltzer® to carbon dioxide, which should be pointed out to students not familiar with stoichiometric ratios. Single trial examples:
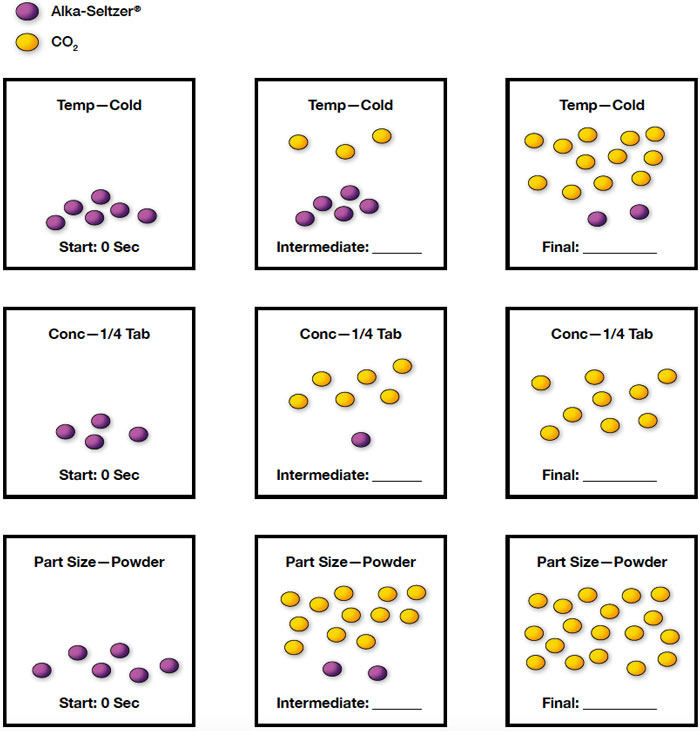
Students investigate the relationship between reactant concentration and reaction rate based on the real-world scenario of a person with acid indigestion consuming an antacid tablet.
We are committed to providing the lab kits, instructional materials, and often free activities and supporting digital resources that do as much heavy lifting for you as we can.



*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it.
Carolina is teamed with teachers and continually provides valuable resources–articles, activities, and how-to videos–to help teachers in their classroom.
Get the latest news, free activities, teacher tips, product info, and more delivered to your inbox.