6-12 Middle/High School
This quick demonstration showing the decomposition of hydrogen peroxide, catalyzed by iodide ions, provides students with visual evidence of a chemical reaction. Students compare the reaction rate of the catalyzed and uncatalyzed reactions and use their data to sketch an energy diagram for the decomposition reaction. The energy diagram serves as a model of energy flow through the reaction, from reactants to products, with 2 different reaction pathways—catalyzed and uncatalyzed.
How does the addition of a catalyst affect reaction rate?
HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
Developing and Using Models
HS-PS1-4: Matter and Its Interactions
Energy and Matter
Upon completion of this demonstration, the bottles can be rinsed, dried, and put away. Keep PPE on in case the bottle contains any leftover hydrogen peroxide.
Unless otherwise prohibited, the foam and catalyst on the trash bag can be carefully rolled up and discarded in the trash.
| Reaction | Time(s) | Observations |
|---|---|---|
| Uncatalyzed Reaction | Will vary with temperature | May see small bubbles |
| Catalyzed Reaction | Instantaneous–runs for about 2 minutes | Large amounts of foam spew out of the bottle |
Hydrogen peroxide (H2O2 ) is stable for at least a year if stored in an airtight opaque container at room temperature. Common in first aid kits, a 3% H2O2 solution can be applied to minor cuts and abrasions. When the solution contacts tissue and blood, it rapidly decomposes into water (H2O) and oxygen gas (O2).
2H2O2( l ) → 2H2O( l ) + O2( g )
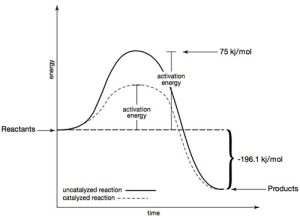
The oxygen gas creates a foam that lifts and washes contaminants out of the wound. This rapid decomposition can only happen in the presence of a catalyst. In the human body that catalyst is catalase, a biological catalyst in blood and tissue. Catalase can lower the activation energy from 75 kJ/mol to about 8 kJ/mol. In this demonstration, solid potassium iodide (KI) dissolves in aqueous H2O2 , forming an aqueous iodide ion (I –) and catalyzing the reaction of aqueous H2O2. The steps of the reaction mechanism are as follows:
Enrich your students’ study of kinetics, catalysts, and decomposition reactions with this unique take on a classic demonstration.
We are committed to providing the lab kits, instructional materials, and often free activities and supporting digital resources that do as much heavy lifting for you as we can.



*Next Generation Science Standards® is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it.
Carolina is teamed with teachers and continually provides valuable resources–articles, activities, and how-to videos–to help teachers in their classroom.
Get the latest news, free activities, teacher tips, product info, and more delivered to your inbox.